|
While not surprising to learn, as most providers are in its vortex, Healthcare is undergoing a massive transformation, and Pharmacy is not immune. As virtually every tenant of patient care has been stretched and reformed as an outcome of the pandemic, reliance on doing things as they have always been done, needs to be reconsidered. Take your medication supply chain for example, pharmacy buyers have had to manage and purchase 3,500 medications which equates to 9,000 different products from over 500 manufacturers while dealing with over 15,000 price changes and 200 plus active drug shortages annually with very poor technology and tools to support their work. Pharmacy buyers jump from website to website to place orders, call in orders, or even fax orders every day. The routine of communicating orders within varied timelines to respective wholesalers and key manufacturers for near real-time resupply in today’s climate requires agility and dynamic responses to be successful. The health system is so overwhelmed it’s been near impossible to get each of the pharmacy buyers aligned on the optimal product for each medication. Variation across the health system is rampant. Opportunity for improvement and cost savings is significant. Regardless of tenure or experience, can a single person or multiple staff manage all the complexities of this process effectively? Is it time for a change, is there a better way?
The opportunity is now for Pharmacy to stand up to these challenges, address these changes, and adopt practices available to affirm the value Pharmacy brings to supporting patient care. Leveraging software and analytics to both automate and direct the procurement process to ensure availability and compliance for acquiring medications, allowing its’ resources to function at top of license, should be a top priority for every health system. To learn more about how Trulla can deliver savings for your health system pharmacy, please contact us at [email protected].
0 Comments
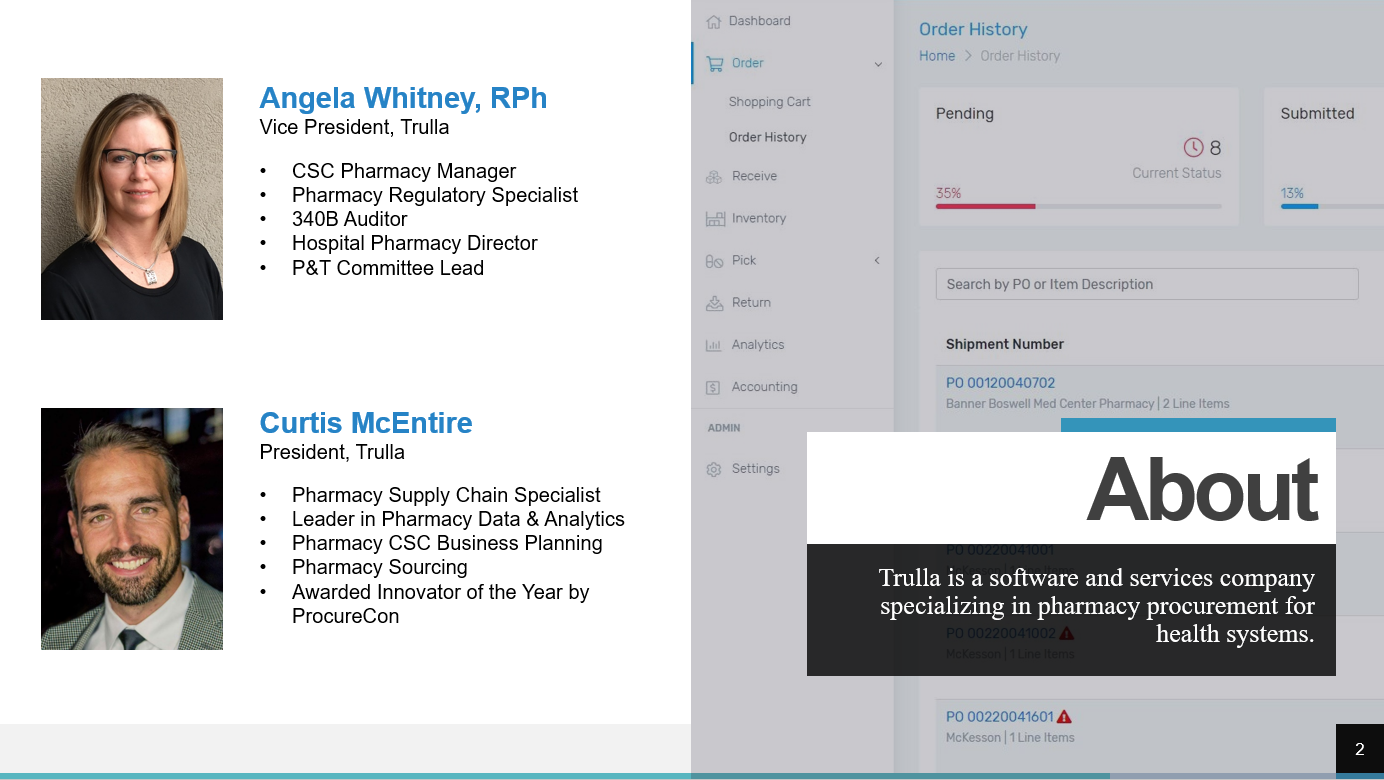
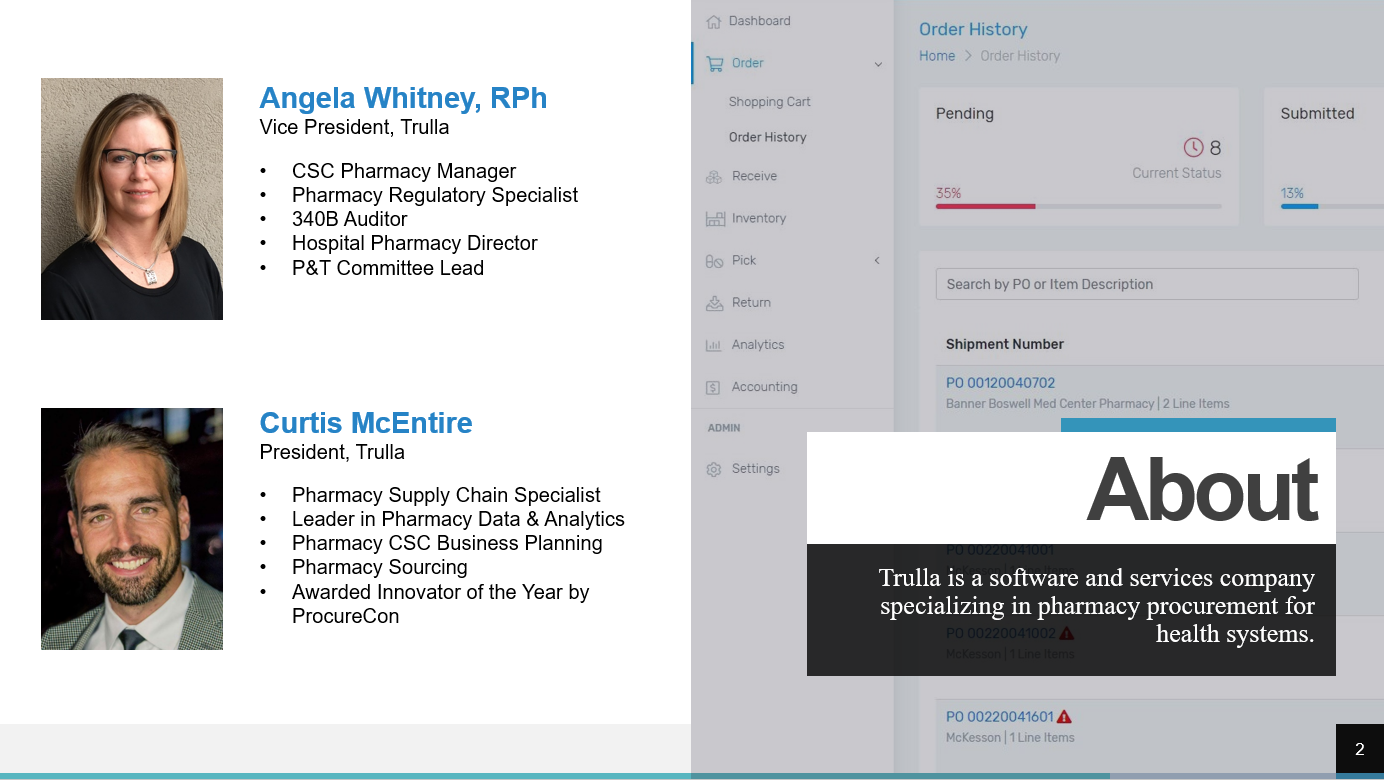
It was the year 2012, I had been working in hospital pharmacies for over 22 years and felt like I had done it all. But then I was exposed to a new challenge: manage the pharmacy operations of our health system’s consolidated service center (CSC). We built our CSC with the purpose of better managing inventory, reducing expired medications, taking advantage of strategic purchasing, repackaging, and sterile and non-sterile compounding. Those all had clear value propositions that we had anticipated. The one activity that completely surprised me was providing pharmaceuticals and pharmacy support to our 300+ clinic locations. Conventionally, clinics receive minimal support and information regarding the medications on formulary, purchasing contracts, suppliers, shortages and management strategies, and the quantities and cost of expired drugs from that location. Often pharmacies within a health system are tasked with supporting the clinics but do not have systems to support this service. Processes are generally developed that minimally meet the need with limited ability to communicate directly with those purchasing pharmaceuticals. Most clinics receive their pharmaceuticals from the health system’s CSC, a nearby hospital pharmacy, or from a distributor like Henry Schein. Take note that it is typical for a clinic distributor to charge a “cost-plus” model while the hospitals are buying the same medications from their primary distributor in a “cost-minus” model. The swing for clinics to purchase most medications through their hospital pharmacy can be close to 10% in savings! For clinic orders to a CSC or hospital pharmacy, the orders often arrive via email, fax, or even a paper requisition that may be hand-delivered to the pharmacy. If the department or clinic is to be charged for the products, then a manual process of entering the transaction information (e.g., drugs, quantities, cost) into a spreadsheet or other program for tracking follows. The transaction information is the submitted to finance periodically to initiate journal entries. At the clinic, drugs are received and accounted for via a few different mechanisms varying in formality. Some clinics simply look through the delivery and restock the drugs while others may be required to sign a paper order to indicate the products were received which is returned to the pharmacy. There is plenty of room for things to slip through the cracks with these manual, disjointed processes. To address the issues described, a health system should consider obtaining a procurement system that will capture cost savings and simplify the ordering and fulfillment processes for their clinics. Finding a solution that addresses both the needs of those procuring pharmaceuticals and those fulfilling the orders can be challenging. Solutions have not historically been aimed at solving this problem. This is one of the reasons we developed Trulla, a pharmacy procurement system built for the entire health system, hospitals, and clinics. Trulla provides valuable information at the point of ordering simplifying the experience for the individual while standardizing purchasing and concurrently capturing cost savings for their clinic. The solution efficiently guides them to purchase the preferred product from the preferred supplier each time with real-time product selection. The ordering process is the same whether the supplier is a hospital pharmacy, a CSC, a wholesaler, or a manufacturer. Trulla can also present a limited list of drugs to the user…only those that they are authorized to buy for patient safety and efficiency, with the ability to create templates easily for even faster ordering. For fulfillment, the system provides pick lists, shipping manifests, and automatic journal entries with updated pricing. For the clinic staff, the receiving process is effortless and supported electronically to improve tracking and accountability. Utilizing a simple, efficient, and cost-effective solution to standardize purchasing for clinics will put smiles on the faces of both the clinic and pharmacy staff and will add time to their day! Angela Whitney, RPhAngela serves as COO for Trulla. She is a 340B ACE and has worked as a Director of Pharmacy, Manager of Pharmacy Operations at a Consolidated Services Center, and other pharmacy roles within a health system. Consolidated Service Centers (CSCs) centralize many pharmacy services along with the inventory of a health system and capture significant savings through processes such as strategic buys, standardization, and low units of measure distribution, just to name a few. But the most frequently asked question by health systems implementing a CSC is: “How can I compliantly ship medications to my 340B covered entities and make it so they can still utilize their available 340B and GPO accumulations?
It becomes increasingly difficult to distribute drugs from a central location when the health system includes one or more 340B covered entities (CEs). Those of us who have worked in the 340B world understand that this is due to the complex rules and regulations covered entities must abide by along with the associated risks if found non-compliant. It is critical for health systems to tackle this problem because the advantages of a consolidated service center are drastically reduced if they are not able to ship products to their CEs. For those of you evaluating the options surrounding central distribution and CEs, or for those of you looking to optimize, please allow us to share some of the observations and lessons learned along the way to becoming the leading team in the industry: Should I just have a separate inventory? Most who consider maintaining separate inventories quickly recognize space will be an issue. There are also serious concerns that multiple inventories would be more difficult to manage and pose a risk that orders will be picked from the wrong inventory, exposing you to 340B compliance violations. A combined inventory is more optimal. Can I just purchase everything at my CSC on WAC? You can purchase all your CSC inventory at WAC both initially and ongoing. The advantage of this approach is that you can compliantly ship to both CE and non-CEs and avoid additional tracking at the CSC. However, purchasing strictly at WAC would significantly increase the cost to the health system, limiting the number of products stocked, services offered, and value of your CSC. What if I purchased everything at GPO? You can choose to just have your CSC purchase strictly at GPO. This inventory could be provided to non-CEs and those CEs that are not subject to the GPO prohibition without additional tracking at the CSC. However, CEs subject to the GPO prohibition would only be able to purchase from the CSC if they have GPO accumulations. For any CE to take advantage of their 340B accumulations and purchase at the lowest cost, they would be required to place orders with their wholesaler or another direct vendor, bypassing the CSC. With this option, the CSC would not be the sole supplier of a product which would impact inventory turns and potentially the product dating. A WAC or GPO based inventory that utilizes the accumulations of the covered entities for replenishment, rather than ignoring them, further reduces overall costs to the health system. Although maintaining a GPO based inventory is clearly the lowest cost, it requires a complex tracking process to maintain compliance. The upside of this approach is that the entire health system can utilize the centralized services and the products stocked at the CSC. CSCs are also able to function as the sole supplier of a product resulting in increased inventory turns, improved product dating, and less waste due to expiration. Everyone who has started down the path of having a GPO inventory with accumulation-based replenishment ends up developing complex manual processes to maintain the compliance of their several CEs. The highest value is with this approach, but the tracking is tedious and next to impossible to manually manage compliance or the inventory levels at the CSC. What is the best solution? Trulla has developed a patent pending procurement solution that enables CSCs to compliantly distribute medications to your CEs while utilizing available 340B and GPO accumulations to maximize their savings. Utilizing a robust 340B compliance engine inside Trulla’s pharmacy procurement software, health systems can maximize savings and ensure compliance when shipping medications from their CSC. Contact us at [email protected] to schedule a demo and learn more. Hospitals subject to the GPO Prohibition can define drugs as NCODs and purchase them strictly at GPO. However, the steps involved in creating a NCOD drug list are not always clear. Below you will find a simplified approach that will guide you through the process.
First, consider whether the manufacturer has entered into a Pharmaceutical Pricing Agreement (PPA) or if the product is classified by the FDA as a device or a vaccine. If the manufacturer has NOT entered into a PPA (i.e., does not offer a 340B price) or the product is either device or a vaccine, then the drug is clearly a NCOD and can be purchased at GPO. These categories of products should be defined as NCODs in policy/procedure and prevented from accumulating and being purchased at 340B. Drugs that are part of/incident to another service and payment is not made as direct reimbursement of the drug (“bundled drugs”) might be interpreted by a covered entity as NCODs under section 1927(k) of the Social Security Act and can be purchased at GPO (see Apexus FAQ 1355). This is based on the statutory limiting “covered outpatient drug” definition under section 1927(k), which might be interpreted by the covered entity as exempting bundled drugs. Therefore, when there is a PPA in place and the product is NOT a vaccine or device, the next step is to determine whether the drug is directly reimbursed. If a drug NOT directly reimbursed and therefore commonly bundled, it can also be considered for inclusion on the covered entity’s defined NCOD list. This evaluation should include a review of several billing claims to determine if the drug appears on claims and whether the NDC is included. When the drug is NOT found on claims or appears as a line item without an NDC, the drug could be considered for inclusion on the NCOD list as this would be considered commonly bundled. If the drug appears on claims along with its NDC, work with your billing department to determine if payors are directly reimbursing. Those products that are directly reimbursed would be difficult to defend as commonly bundled. Work closely with your billing department to align charging practices and NCOD determinations. Furthermore, clearly define NCODs in policy/procedure and provide a defensible position based on the covered outpatient drug and limiting definition of section 1927(k) of the Social Security Act. When operationalizing, be certain to consistently apply the NCODs definition across all registered sites, including clean sites and ensure auditable records are maintained. Don’t forget to adjust your third-party administrator settings to prevent them from accumulating and being purchased at 340B. If your health system has a consolidated service center (CSC), ensure that they have mechanism to track, manage, and purchase the drugs on each covered entity’s NCOD list correctly. Are you considering a pharmacy CSC (consolidated services center) for your health system? This recorded webinar dives into what you need to know when considering a pharmacy CSC. Learn what services you should consider, challenges o watch out for, 340B complexities, licensure, and more!
This webinar is presented by Angela Whitney, RPh and Curtis McEntire, who have been integral in the planning and operations of multiple pharmacy CSC's around the country. Click here to access the webinar. One of the most frequently received questions relating to a Consolidated Services Center Pharmacy (CSC) is simply to know what the benefit is. Why go through all the labor in formulating, presenting, and executing a CSC plan? We totally understand that, on the surface, there can easily be some ROI doubts, but there are a few overarching ways that a CSC can add value: Cost Savings, Improved Patient Safety, and Process Improvement. This article will focus on the qualitative benefits that can come with implementing a Pharmacy CSC at your health system.
Due to many facilities sharing the cost of a CSC, the health system can more readily afford better facilities, better equipment, additional training, and be able to perform testing that could not easily or practically be conducted at most locations. For example, we were able to conduct stability studies and sterility testing of the products compounded by our CSC Pharmacy. This allowed us to be confident in the quality of our products that we were providing to our patients. We also found it substantially easier to implement consistent processes, procedures, and to increase the expectations of staff. The CSC team is a small group of highly trained individuals residing in a single location as opposed to being spread across the health system. Our CSC team had daily huddles to share information quickly and easily to proactively identify and institute process improvements. Due to increased expectations and training, the team members are more often than not, Specialists, and further raise the bar by holding each other accountable. Amongst our team, there was a unified commitment to exceed expectations and avert anticipated problems. A CSC Pharmacy can ensure a consistent supply of high-quality medications at the lowest possible cost, minimizing the impact of drug shortages and maximizing best practice. For example, our CSC had a three (3) month supply of propofol. This allowed us to avoid purchasing off contract and to continue business as usual, performing ambulatory surgeries and other procedures requiring sedation, when the propofol shortage was in full swing. A CSC can also maintain an inventory of antidotes, disaster medications, and other high cost medications needed for patient care so that the health system is better prepared while reducing the inventory burden of each facility. We synchronized the antidote inventory levels of the CSC and each facility by considering things such as distance from the CSC, accessibility of couriers, and amount required to have on hand for the initial dose(s). These types of efforts also resulted in a reduction of expired medications and freed up valuable space at the inpatient pharmacies. A CSC Pharmacy reduces the transactional work at the facilities, allowing clinical staff to focus on clinical service, patients, and site-specific activities, as they should! However, we immediately identified an unanticipated benefit of the CSC. Undeniably, there were far less interruptions in this controlled environment when compared to an inpatient pharmacy. At times, an inpatient pharmacy can be chaotic with new orders, missing dose requests, clinical questions, and other urgent situations (e.g., stroke calls, codes, rapidly declining condition of a patient, etc.). In contrast, the CSC is relatively calm from an operations perspective. Team members dedicated to compounding and packaging were able to focus on the task at hand, without competing priorities, which should result in a reduction in medication errors and an improvement in Patient Safety. Albeit reducing medication errors and improving Patient Safety is a primary objective of pharmacy services and a significant value add, a CSCP also increases efficiency, decreases service interruption, and results in an overall improvement in the drug supply. These improvements bring about unrecognized Cost Savings for the health system. When a Consolidated Services Center is being considered, it is our job to bring this to the attention of our leaders and to shed light on ALL the benefits of a Pharmacy CSC! Imagine walking through the doors of your hospital and the floors are covered with $100 bills and all you need to do is pick them up. Saving money in your hospital pharmacy spend is about that easy if you know what you’re doing, and you have the right tools to pick up the dollars. Over the last decade I have worked with countless hospitals and health systems to help optimize their pharmacy purchasing. Pharmaceutical spend within a health system is usually the largest or second largest category of annual non-labor spend within a health system. It consists of thousands of medications, even more NDC’s, hundreds of contracts and manufacturers, and dozens of suppliers. It can be one of the most daunting categories to try to optimize and capture cost savings. But…if you know what to look for and have the right tools, finding and capturing the opportunities in this category will result in the most significant savings for your health system. It all starts with one foundational element: really strong pharmacy spend analytics. I’m not talking about the basic, canned reports provided by your wholesaler or group purchasing organization (GPO). You have had those same reports for two decades. Sure, they’re helpful in giving you some high-level spend numbers or even the details of a specific invoice. But in most cases, despite those reports, the savings are right there at your feet…you just can’t see them. I’ve been building analytics specifically for pharmacy purchasing for a long time. When I first started, my reports were basic. Show me total spend over time. Check. Show me total spend by therapeutic class. Check. Things like that. But as I learned more about pharmacy, the medications, and the data, I started to see the thousands of dollars on the floor that we were just walking over every day. All we had to do was pick it up. That’s when pharmacy analytics began to change for me. I began stitching together data in such a way that the largest and easiest savings opportunities rose right to the top. Work became much easier to prioritize. “Potential Savings” quickly and easily became “Realized Savings!” And not just in the hundreds of thousands of dollars, but in the millions of dollars. In a single year, one of my health system clients saved over $30 million based upon simple and clear recommendations from my spend analytics! I am not surprised as I meet with new clients that they do not have much in the realm of good pharmacy spend analytics. So, don’t get down on yourself if you don’t; that’s the case for the majority of hospitals and health systems around the country. But do not let it stay that way. The money is at your feet. Make changes now to start saving now. Implement powerful pharmacy spend analytics and start picking up the money. Reach out to us at [email protected] to learn more about how we can help your hospital and health systems pharmacies start saving significant dollars today! AuthorCurtis McEntire. CEO of Trulla. Curtis is a pharmacy supply chain expert and is dedicated to helping health systems optimize their pharmacy supply chain. How much does the drug you’re dispensing actually cost and are you buying it at the best possible price? Did you know that for every medication that is generically available there is an average of 13 NDC’s that can be purchased? With drugs and drug prices constantly changing, knowing which NDC to order and utilize is complex for even the most experienced pharmacy buyers. Contractual agreements supported by rebates, administrative fees, or 3rd party programs can cloud what on the surface seems like an easy question – is this the NDC I should buy? In pharmacy, it’s so complex to analyze and review the true pricing, not to mention trying to couple that analysis with current and historical utilization, tier thresholds, and other variables. It’s even harder when you consider that most of the “tools” we do have access to are in multiple, disparate, manual solutions. Add in the variables of the 340B Program requirements and other clinical-based programs and you soon realize how difficult, if not impossible, it is to guide purchasing within even the smallest inpatient pharmacies, let alone across an entire health system, a growing number of which include a central distribution operation. The tragedy is that we all understand that standardizing to the best NDCs and suppliers for your organization can bring significant benefits both clinically and financially. Having a software solution which can ingest utilization and purchasing data from your suppliers and communicate with those suppliers can sharply improve the benefits for an organization. Imagine being able to identify and control the NDCs to order at a health system level by leveraging the preferred logic and the settings available in the Trulla Procurement Software. This ability will drive several benefits to the health system which includes:
By leveraging the analytics derived from current spending patterns and the software which can set default, preferred NDCs, Trulla is uniquely positioned to identify your best opportunities and deliver results by allowing leaders to easily implement decisions across your health system. The impact of Trulla is amplified as health systems become increasingly complex. Trulla’s software is specifically designed to enable the selection of preferred NDCs to meet the needs of corporate and/or local initiatives and to continuously guide users throughout the procurement process. Finally, the industry has a tool to not only direct you to savings within your pharmacy spend, but to implement decisions and track the benefits they deliver! Isn’t it time to maximize your pharmacy savings? Formulary management. There are few words found within the pharmaceutical lexicon that elicit as much joy and exuberance as “formulary management”. If you think that’s even remotely true, you must be new to formulary management. With the (thankfully) increasing opinion that a health system should operate more like an actual system, the dire need for proper and auditable formulary management is becoming increasingly evident. We can’t run a diverse health system with a one-size fits all approach and we also can’t efficiently serve our patients if we persist in the historical “every facility an island” approach. Across the industry, we just don’t see good auditing tools to ensure that we’re purchasing the correct, optimized pharmaceuticals. We’ve spoken previously about the unsung hero, the pharmacy buyer, and we can really give them and their clinic counterparts a leg up by handing them a tool that ensures proper formulary management and compliance.
There are lots of reasons why system-wide formulary management is complicated: hospital vs. clinic, patient populations, the 340B Drug Pricing Program, different regional needs, supplier access, and so on. The list of why there hasn’t been a good solution to this is much shorter: no one that truly understands the industry has built a comprehensive tool for the industry, until now. Trulla enables users to operate from the broadest sense (all active medications for an entire health system) to the absolute most narrow sense (the 15 medications that a seasonal shack on the backside of a mountain might need access to). The way that Trulla approaches formulary management is novel in a few ways, not the least of which is that we recognize and respect that there are medications that a health system may need that they don’t want everyone purchasing at will and that the pharmacy buyer can never be replaced; they need the freedom to be able to purchase beyond machine-driven logic. Trulla operates as a funnel to present a buyer with the optimized product, specific to their department and health system, but we don’t lock them into that product. At the widest point of the funnel, we have access to all active medications with the FDA so that those managing the formulary don’t have to build each medication profile from scratch. The next step in the narrowing funnel includes on formulary and off formulary medications, restricted medications, and so on. As we narrow the funnel even more, Trulla allows for the restriction of a visible sub-formulary as it pertains to each buyer and their location. Let’s say there’s a system-level decision made by the P&T Committee, or the 340B team has new information about where to purchase X medication from to realize the best compliant price, Trulla allows for the operationalizing of that information. A 340B clean site will see only those medications that they should have rights to purchase. A non-340B clinic may see a broader sub-formulary but still doesn’t have access to everything an inpatient pharmacy would have access to, and so on. Proper formulary management, coupled with a tool to operationalize it, creates an environment where even the administrative assistant at a clinic who’s thrown into a one time only purchasing situation can see the same results as the most experienced hospital buyer. Your goal is to save time and money while keeping patient safety at the forefront of every decision. Formulary management may not be the first thing that comes to mind when thinking about saving time and money, but let Trulla show you a new angle that’s afforded by our software. With the Trulla software you’ll have access to a ready-to-go, customizable medication list to begin building a broad formulary that serves as a source for any number of sub-formularies for those who purchase pharmaceuticals. With our tools, you’ll have a united front all the way across your facility or health system, working together towards the same goals. Many healthcare systems are creating Consolidated Service Centers (CSC) to help standardize the pharmaceutical experience throughout their system. There are many cost savings and quality improvement benefits to consolidating pharmaceutical practices. Unfortunately, when it comes to centralizing sterile compounding there is a great deal of misunderstanding when it comes to registering with the FDA; specifically, whether to be a state regulated 503A or an FDA regulated 503B. The most common notion is that registering as a 503B is the right approach. Even though that approach might appear to be the safest option, registering with the FDA as a 503B outsourcing manufacturer is not the same thing as operating an inpatient pharmacy following the standard USP 797 regulations. It is better to think of 503B as becoming an actual manufacturer with its own set of rules. To be effective, selecting the 503B path would require a different business model to cost justify such an operation. Fortunately, working within the confines of the 503A classification can still produce a quality product for patients and help keep the high costs associated with Current Good Manufacturing Practice (CGMP) from disrupting certain aspects of a consolidated services approach.
The draft guidance for Hospital and Health System Compounding Under the Federal Food, Drug, and Cosmetic Act: Guidance for Industry dated April 2016 states, “…FDA does not intend to take action if a hospital pharmacy distributes compounded drug products without first receiving a patient-specific prescription or order provided that: (1) The drug products are distributed only to healthcare facilities that are owned and controlled by the same entity that owns and controls the hospital pharmacy and that are located with a 1 mile radius of the compounding pharmacy;” Forcing CSCs to register as 503B because they move compounded product more than 1 mile from the facility that compounded the product is frequently misinterpreted and does not align with the regulations which clearly allow for anticipatory compounding and interstate distribution of products when certain conditions are met. It is important to focus on the applicable statutes and regulations as found in Section 503A of the Federal Food, Drug, and Cosmetic Act. The guidance documents have limited ability to be enforced when they make statements and claims outside of the applicable statutes and regulations. To reinforce this, many of the Guidance for Industry documents contain in their introductions: “You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations.” The headers on many of the pages within the documents also state: “Contains Nonbinding Recommendations.” The key requirements for maintaining 503A compliance for most CSCs are as simple as:
CSCs are in a unique position to receive valid prescription orders for identified individual patients. With the advancements in electronic health records, CSCs can operate as a member of their health system with full access to required documentation and the ability to generate auditable reports to support their practice. Section 503A and the FDA guidance documents have left opportunities for healthcare systems to take a defensible position on identifying their CSC(s) as 503A. Consolidating sterile compounding can increase safety and decrease costs for patients without taking on the unnecessary burdens of registering with the FDA as a 503B outsourcing manufacturer. |
ARCHIVES
March 2022
Categories
All
Disclaimer: The information provided in this article does not constitute legal advice and should not be construed as such. Readers of this document are encouraged to contact their attorney to obtain advice with respect to any particular legal matter. The views expressed in this document are those of the author and not those of the Trulla LLC. All liability with respect to actions taken or not taken based on the contents of this document are hereby expressly disclaimed. The content in this document is provided “as is;” no representations are made that the content is error-free. |
Contact us to help you optimize your pharmacy procurement. |
E-mail: [email protected]
|







 RSS Feed
RSS Feed